Your basket
Your basket is currently empty.
Tampon Case for 4 tampons
-£1
ProViotic - 30 tablets
-£1

HPV, STIs, vaginal microbiome
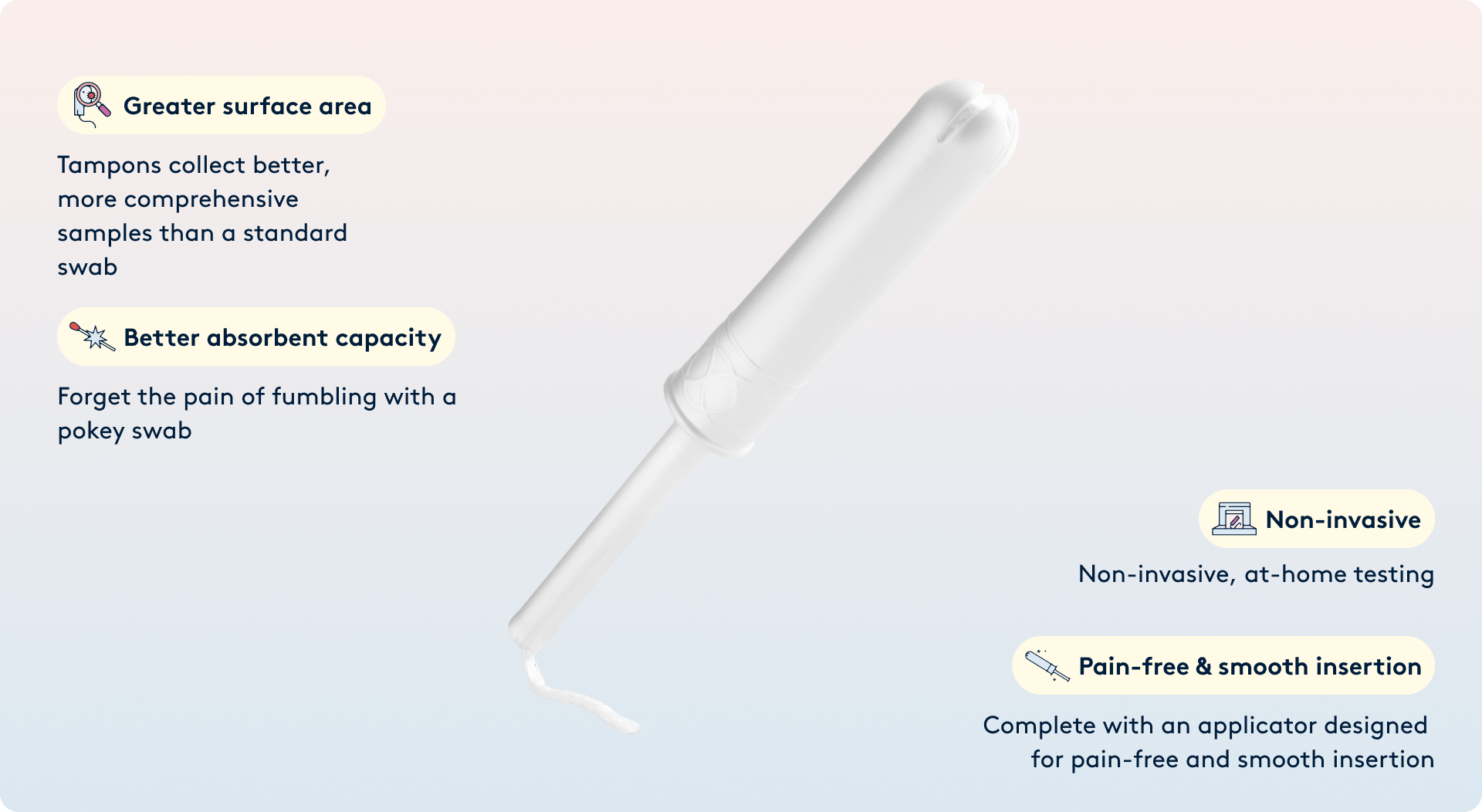
Diagnostic Tampon
The Daye Diagnostic Tampon is a CE-marked, self-sampling medical-grade tampon designed to collect high-quality vaginal and cervical fluid samples for laboratory analysis. Samples can be analyzed for high-risk HPV, selected STIs, and vaginal microbiome markers (depending on regional availability), all from the comfort of home, with clinically validated accuracy.
How it works
1
Insert the Diagnostic Tampon for ~20 minutes.
2
Remove and post using the prepaid envelope.
3
Our partner labs run validated qPCR assays.
4
Users receive a structured report and clinical next steps.
Why tampons for sampling
- Full-coverage sampling - Daye tampons cover the entire vaginal canal and reach the cervix, providing a larger surface area and longer contact time than swabs - capturing more vaginal material for analysis.
- Improved sample validity - Comparative studies show higher valid-result rates than clinician-collected swabs or self-collected vaginal swabs.
- Familiar format - Many patients prefer tampon-based self-sampling due to the device’s familiar nature, leading to higher participation and comfort levels across varied demographics.
One tampon, multiple panels
Evidence highlights
Analytical performance has been evaluated in randomised control, statistically significant comparative studies against clinician-collected and self-collected swabs.
HPV detection performance vs clinician-collected swabs (CCS)
In a head-to-head comparison, the Daye Diagnostic Tampon demonstrated sensitivity of ≈ 82.9% and specificity of ≈ 91.6%, relative to clinician-collected swabs, yielding an overall accuracy of ~89%. The valid result rate was 99.2% (versus 95.4% for self-collected vaginal swabs and 90.8% for clinician swabs). [study 1], [study 2]
STI detection
In a trial comparison, the Daye Diagnostic Tampon correctly identified the positive samples, with 100.0% sensitivity and specificity of 100.0% relative to clinician-collected swabs. [study 5]
Microbiome Profiling
Why this matters?
0.0%
HPV detection performance vs clinician-collected swabs
0%
in one cohort were willing to provide a tampon sample
0%
Chlamydia & Gonorrhea; Bacterial Vaginosis (BV): In a trial comparison

Available Panels
The results can provide information on the presence of specific pathogens, viruses, or patterns of vaginal microbiota associated with conditions such as BV, preterm birth, or infertility. This information may support clinical decision-making when interpreted by qualified healthcare professionals.
Persistent infection with high-risk HPV types is the necessary cause of almost all cervical cancers. Detection of high-risk HPV informs risk stratification for cervical screening and may indicate the need for further investigation (e.g., cytology, colposcopy) as per national guidelines.
- HPV-16, HPV-18 – highest oncogenic risk; responsible for ~65 % of cervical cancers.
- HPV-31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 – additional high-risk types associated with high-grade cervical intraepithelial neoplasia (CIN2/3) and cervical cancer progression.
- Common STIs can cause acute symptoms and long-term reproductive complications if left undetected and untreated, including pelvic inflammatory disease (PID), reduced fertility, and adverse pregnancy outcomes. Chlamydia trachomatis (CT) – most common bacterial STI globally. Neisseria gonorrheae (NG) – can lead to PID, reduced fertility, neonatal complications. Trichomonas vaginalis (TV) – associated with vaginitis, increased HIV acquisition risk, adverse pregnancy outcomes. Mycoplasma genitalium (Mgen) – emerging STI linked to urethritis, cervicitis, PID, and reduced fertility.
- The balance of the vaginal bacteria is increasingly recognized as an important factor in gynecological and reproductive health. [1] Microbiome results can indicate the presence of imbalances or specific microbial patterns associated with conditions such as BV, yeast infection, or adverse reproductive outcomes. Lactobacilli – “good bacteria”, maintain acidic vaginal pH and inhibit pathogen overgrowth, protecting against infection. Anaerobic bacteria (Gardnerella vaginalis, Prevotella bivia, Porpwyromonas) – disrupt vaginal balance, associated with BV, miscarriage, preterm birth, increased STI risk. Candida spp. – yeast species that can cause yeast infection. Mycoplasma hominis – may contribute to dysbiosis, PID, and preterm birth when abundant and symptomatic. Ureaplasma urealyticum / Ureaplasma parvum – opportunistic organisms linked to reproductive complications at high loads.
Did you know?
High valid-result rate = no repeat sampling.
How the panels work together
Your vaginal and reproductive health are shaped by connected biological systems — viral, bacterial, hormonal, and immune.
HPV
Microbiome
Persistent high-risk HPV infection can lead to cervical cancer, but not all infections progress. Research shows that a Lactobacillus-dominant vaginal microbiome supports HPV clearance, while imbalanced microbiomes increase the risk of persistence and progression.
STI
Microbiome
Certain STIs can alter the vaginal microbial ecosystem, while imbalance can increase susceptibility to STIs. Measuring both simultaneously can help distinguish between primary infections and secondary shifts in microbial balance.
HPV
STI
Co-infection with STIs can influence HPV persistence and progression. For example, Chlamydia trachomatis infection has been linked with higher rates of HPV-related cervical abnormalities.

Regulatory & Quality
In‑vitro diagnostic testing performed by UKAS‑accredited, CQC‑registered partner laboratories. Diagnostic Tampon CE and UKCA-marked as a sterile medical device under MDR. All analyses are conducted using CE-marked qPCR assays. Results must be interpreted by a qualified professional. This at‑home sample collection device is not a substitute for cervical cytology/colposcopy. HPV positive patients are referred for cytology.



*Samples are processed in accredited laboratories under strict quality controls. Data are handled in compliance with GDPR and HIPAA standards.
Published research
1.Efficacy and acceptability of a self-collected medical grade tampon as a novel vaginal sample collection tool for the detection of HPV and STIs
Center for Applied Scince and Innovation
This study aims to compare the efficacy and suitability of a self-collected tampon for the detection of human papillomavirus (HPV) and sexually transmitted infections (STIs) using qualitative TMA-based assays
Can samples taken with Daye tampons be used after being freezed?
University of Liverpool
There’s no statistically significant difference between corresponding fresh and frozen samples, enabling the conclusion that samples can be frozen before processing. The abbility to freeze tampon samples allows for processing at a more practical time.
3. Menstrual Tampons Are Reliable and Acceptable Tools to Self-Collect Vaginal Microbiome Samples
Center for Applied Scince and Innovation
Menstrual tampons are a more effective tool for sample collection compared to the vaginal and cervical swabs. There are confirmed similar results in literature with reports of high specificity with variable sensitivity.
4. Efficacy of Self-Collected diagnostic tampon as a Novel Tool for Vaginal Sample Collection Towards the Detection of Genital Infections
Institute for Medical Research
This study aimed to assess the suitability of self-collected diagnostic tampons as a specimen collection device for analyzing the vaginal microbiome using qualitative real-time polymerase chain reaction (rtPCR).





