Your basket
Your basket is currently empty.
Tampon Case for 4 tampons
£-1
ProViotic - 30 tablets
£-1

The rigorous science behind Daye tampons
We are committed to raising the bar in Gynae health. We invest heavily in research and development with the aim to bridge the gap in medical innovation. Our cannabinoid-coated tampons were our first step in our journey towards building a more comprehensive and holistic health platform.
Disclaimer: These claims have been reviewed by Daye’s Scientific Advisory Board, but not the MHRA or the FDA. This page is not intended for marketing, but for research and scientific purposes only.

Daye tampons are proven to be safe
We follow the golden standard in clinical trial design and post-market surveillance to ensure the safety and suitability of our tampons. Here’s how we did it:

Placebo-controlled trials

Double blinded study design

Monthly vaginal screening on all participants

80 participants
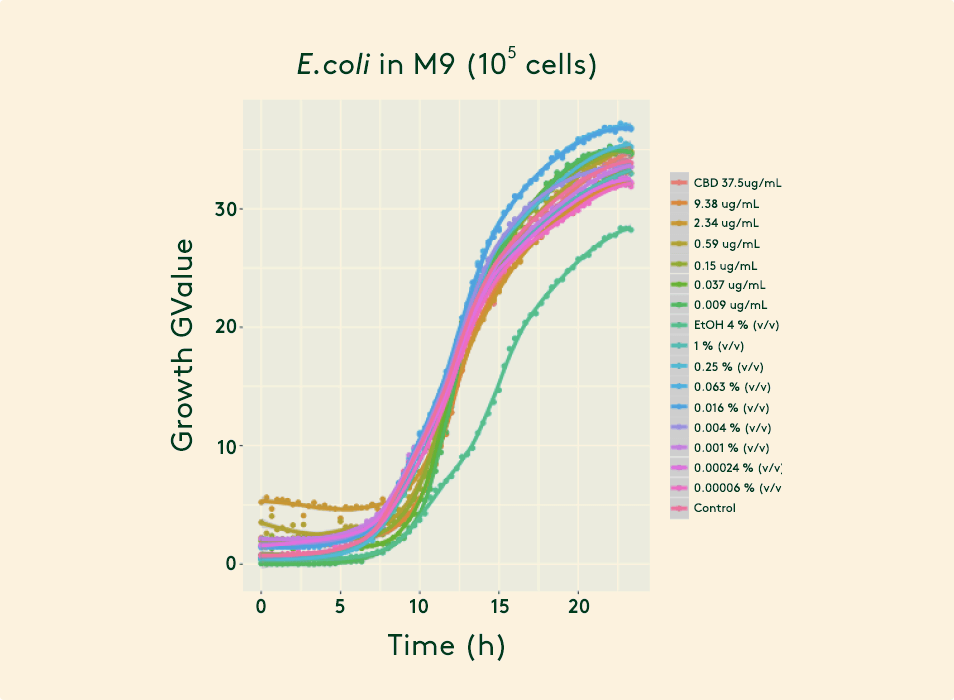
Impact on the vaginal microbiome
The study has concluded that Daye tampons do not impact the vaginal microbiome negatively. In fact, the CBD was shown to increase the level of good bacteria (Lactobacilli).

TSS-associated toxin production
No detectable Toxic Shock Syndrome toxin was found on the Daye tampon after 8 hours of incubation.
Daye tampons are extensively tested
At Daye, we take your vaginal health seriously. In addition to our clinical trials, we have also conducted a number of biocompatibility studies to prove the long-term safety of our tampons.

Vaginal Irritation
CBD tampons did not cause any irritation to the vaginal mucosa.

TSS Risk Reduction
Daye’s CBD tampons reduced the production of TSS-causing toxins.

Toxicity
The tampons did not cause subacute or subchronic toxicity.

Abrasions of the Vagina
No abrastions to the vaginal walls have been detected during our studies.

Impact of CBD on Good Bacteria
CBD tampons had a positive impact on Lactobacilli growth, strengthening the vaginal microbiome.

Acute Systemic Toxicity
This study verified that CBD tampons do not systemically cause organ damage.

Material Mediated Pyrogenicity
The study has deemed that CBD tampons are free of pyrogenic substances which could cause fevers.

Ongoing Quality Control
Every part of our supply chain is rigorously tested. We ensure that Daye tampons are free from microbial contamination, fragments of heavy metals, and that they have just the right amount of CBD — with absolutely no THC.

Candida

Not present

E. Coli

Not present

Staph

Not present

Arsenic

Not present

Lead

Not present

Mercury

Not present

Dioxins

Not present

Pesticides

Not present

CBD

30%

THC

0%
Disclaimer: Human clinical trials have been completed and reviewed by independent clinicians as well as the above. At present, regulatory authorities mandate that only prescription drugs should make their clinical evidence available to the general public. As we are not regulated as a prescription drug, we do not make our studies available publicly, so we can stay compliant with regulations. If you are a researcher and you'd like to review our data, please contact our customer care team and we'll send you a data pack.
Real-life Efficacy Data
We are committed to radical transparency, and collect more Post-Market Surviellance (PMS) data, than most pharma companies out there. Our data is reviewed by an independent board of Medical Advisors, so we can continue to ensure the quality, efficacy and safety of our tampons.

How likely are you to refer Daye to a friend?
9.3 average based on 1928 total responses
Pharmaceutical-grade cannabinoids
The lack of regulation in the cannabinoid industry means that poor quality products have become the market standard. At Daye, we’re changing that and only use cannabinoids produced to pharmaceutical-grade standards. This means:

CO2 extraction
An extraction method which removes the need for solvents, which in turn ensures the end product is free from chemical residues.

THC-free
Our exclusive supplier has a patented technology for removing all THC from the final CBD extract. So no, our tampons will not get you high, and yes, you can go through TSA with them.

Clean production
Closed-loop + stainless steel production: all our machine components are made of stainless steel. The production is fully isolated from air and outside contamination.

Single supplier
Our exclusive CBD supplier owns the entirety of their supply chain — from farm to bottle, ensuring they have unprecedented quality control over the final CBD extract.

Medical Board
Our medical board comprises clinicians, researchers, and OBGYNs across the US, EU, and UK.

Dr Baijja Raimi-Abraham
Pharmacist, Lecturer in Pharmaceutics at King’s, Founder of King’s College London Fight the Fakes and Academic Lead

Dr Belinda Coker
Experienced GP and Medical Director with 20 years of expertise in fertility, women's health, customer experience and clinical quality

Prof. Stephen K. Smith
Held senior positions in Academic Medicine and the NHS at the University of Cambridge, Imperial College, London and the University of Melbourne

Dr Jack Pearson
Medical Affairs Manager at Natural Cycles, the only digital method of birth control cleared by regulator both in the US and in Europe

Dr. Melanie Bone
ACOG certified OBGYN, who has adopted CBD in her practice

Prof. Dharani Hapangama
Professor of Obstetrics and Gynaecology at the University of Liverpool, with a research interest in gynaecological cancers

Dr. Nicola Tempest
Senior Registrar and Clinical Research Fellow with an interest in endometriosis, Liverpool Women’s Hospital

Peter Hutt
Former General Counsel of the FDA

Dr Paula Briggs
Chair of the British Menopause Society, Consultant in Sexual & Reproductive Health at Liverpool Women’s Hospital

Dr Anatole Menon- Johanson
Harkness Fellow and Sexual and Reproductive Health Department Co-Lead at Guy's and St Thomas' NHS Foundation Trust

Dr Patrick Kimmitt
Senior Lecturer at the University of Westminster, with broad and diverse range of research interests within medical microbiology and molecular biology

Peter Galen
Chief Innovation Officer at Hemex Health, Inc — a company that contributes to health equity across the world by providing innovative, smart solutions that make advanced technology affordable and practical

Prof. Louise Kenny
Executive Pro-Vice Chancellor, with an interest in pregnancy disorders, Liverpool University

Prof. Douglas Kell
Research Chair in Systems Biology, University of Liverpool

The Daye Gold Standard
We're committed to raising the standards in gynae health products and innovation by investing in research and development.

ISO13485 is the global auditing framework for the quality of medical devices and pharmaceuticals, issued by independent bodies that inspect our clinical data, batch testing, and factory operations.

The CE mark is the European quality standard for the design, manufacture, and clinical validation of medical devices.

GMP confirms that the company’s production and quality standards fall in line with regulations for medical devices after an independent audit.





