Your basket
Your basket is currently empty.
Tampon Case for 4 tampons
£-1
ProViotic - 30 tablets
£-1

HPV Screen
Detect 14 high-risk HPV infections with our non-invasive at-home tampon screening kit.
Taking your sample is super simple. Just insert the Daye tampon, wear it for at least 20 minutes (or up to 8 hours if you prefer), then remove it. You should not be on your period, so the test works whether you're menopausal, pregnant, or using hormonal contraception.
After collecting your sample, send it back to us in the prepaid packaging. Our lab will analyse it using advanced PCR technology.
Within 5-10 days, we'll have your results ready in your secure online account. You'll receive a comprehensive breakdown of what we found, along with clear explanations of what it all means for your health.
If you test positive for HPV, our team of nurses will invite you for a free 15-minute consultation to discuss your results, answer any questions, and guide you through your next steps.
For any other results that need attention, you can easily book a consultation with a healthcare specialist or access appropriate treatments—all through your Daye dashboard. We've designed the whole process to be straightforward, private, and completely on your terms.
No, Daye's HPV screening is NOT a replacement for NHS cervical screening (smear tests). You should still go to all your regular NHS screening appointments. The NHS test is vital because it takes cells directly from your cervix, spots any abnormal cells that might become cancer, allows doctors to treat these cells early, and has been shown to lower cervical cancer rates by up to 70%.
Daye's HPV screening works alongside this as an easy at-home test using a tampon. Our test checks for 12 high-risk HPV strains, including HPV 16 and 18 (which cause most cervical cancers). Since nearly all cervical cancer (99.8%) is caused by HPV, keeping track of your HPV status is a helpful way to catch potential issues early. Our test lets you check for HPV between your NHS appointments (which only happen every 3-5 years), helps you find out if you have high-risk HPV sooner, and shows whether an infection has cleared on its own.
If you test positive with our screening, remember that HPV is very common and doesn't mean you have cancer. You should still go to your next NHS screening appointment to check if HPV has caused any cell changes.
The vaginal microbiome comprises a group of microbes residing in the vagina, including Lactobacilli, the beneficial bacteria that protect against HPV infections. Lactobacilli produce compounds that combat harmful bacteria, reducing infections and assisting in the natural elimination of HPV. Conversely, an imbalance with harmful bacteria (such as bacterial vaginosis or BV) can facilitate HPV persistence. Maintaining a healthy equilibrium of the vaginal microbiome enhances the body's capacity to naturally eliminate the HPV virus.
Daye uses tampons for at-home screening because they provide a non-invasive, familiar, and comfortable method for patients to collect comprehensive samples from their entire vaginal canals. Studies published in the Journal of Human Reproduction and BMC Women's Health have shown that Daye tampons offer improved sensitivity for detecting infections compared to the traditional swab. By using a tampon for at-home screening, you can trust the sample you have collected is reliable.
Frequently bought together
Who is this service for?
If you had HPV in the past
If you had HPV in the past
Track if your HPV infection persists to help prevent the development of cervical cancer.
We suggest screening once every year to monitor your health.If your busy lifestyle gets in the way
If your busy lifestyle gets in the way
At-home testing for HPV with a tampon is a convenient, easy way to stay on top of your gynae health.
We suggest a one-off screen to identify all treatable issueIf you find in-clinic exams invasive
If you find in-clinic exams invasive
The Diagnostic Tampon is designed to be a discreet and non-invasive way to detect HPV from the comfort of your safe space.
We suggest 1-2 screens per year, and anytime you have symptoms.The Human Papillomavirus (HPV), mainly transmitted through sexual contact, causes nearly all cervical cancer cases. Studies suggest around 80% of people will contract HPV at some stage in life, often without symptoms.
Which pathogens do we test for?
With cutting-edge PCR technology for unparalleled accuracy, Daye tampons collect more vaginal fluid than a standard swab and cover the whole vaginal canal.
HPV screening
Detects the presence of high risk human papillomavirus strains.
*Only available with the HPV Screen
Lactobacilli
Good bacteria in your vaginal microbiome.
Anaerobic bacteria
Cause infections like bacterial vaginosis (BV).
Candida
Candida is a fungus which affects 75% of women.
Mycoplasma/ Ureaplasma
According to studies, can affect fertility.
Chlamydia
A very common STI caused by a bacterial infection.
*Only available with the STI Screen
Gonorrhoea
An STI that can affect both men and women.
*Only available with the STI Screen
Trichomoniasis
One of the most common STIs worldwide.
*Only available with the STI Screen
Herpes Type I & II
Can potentially infect another person.
*Only available with the STI Screen
What is included with every screening?
Order your test & get a kit delivered to your door
The kit will arrive at your doorstep in a discreet package. No more awkward queuing in clinic waiting rooms.

Collect and send off your sample to our accredited lab
Activate your kit with your unique code, complete a short symptom questionnaire, and send your sample - we'll handle the rest.

Get detailed, actionable results.
We never want to leave you stuck with a set of confusing or potentially troubling results. If you test positive for an STI, one of our sexual health nurses will provide a free consultation.
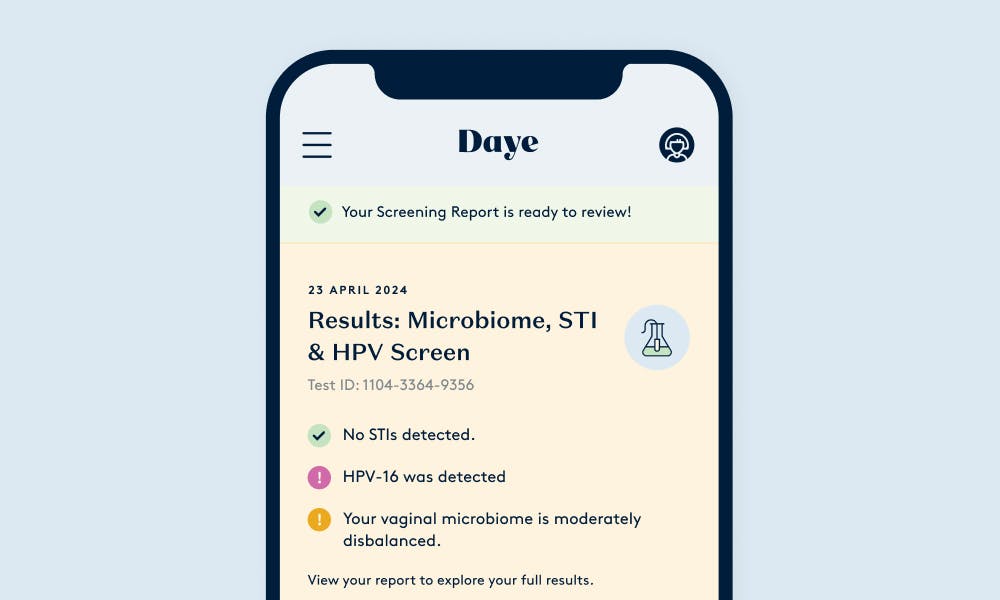
Connect with vetted specialists & get prescription treatments to your doorstep.
After the screening, you can set up virtual consultations with our vetted sexual health, fertility, and menopause experts. You can also get treatments discreetly delivered to your door.

Discover simple changes you can implement to take control of your health
Every report includes science-backed, personalised insights into lifestyle changes you can adopt to further enhance your health.

Be your own health hero
Got a recurring infection?
Got a recurring infection?
Mainstream treatments for BV and thrush can put us at a higher risk of recurrent infections.
Learn moreOwn your fertility journey.
Own your fertility journey.
A healthy microbiome is vital for successful fertilisation, implantation, and embryo development.
Learn moreWorried about STIs?
Worried about STIs?
As many as 70% of female STIs are asymptomatic, making proactive screening essential.
Learn more
Developed by experts
Our collaborator in advancing biotechnology
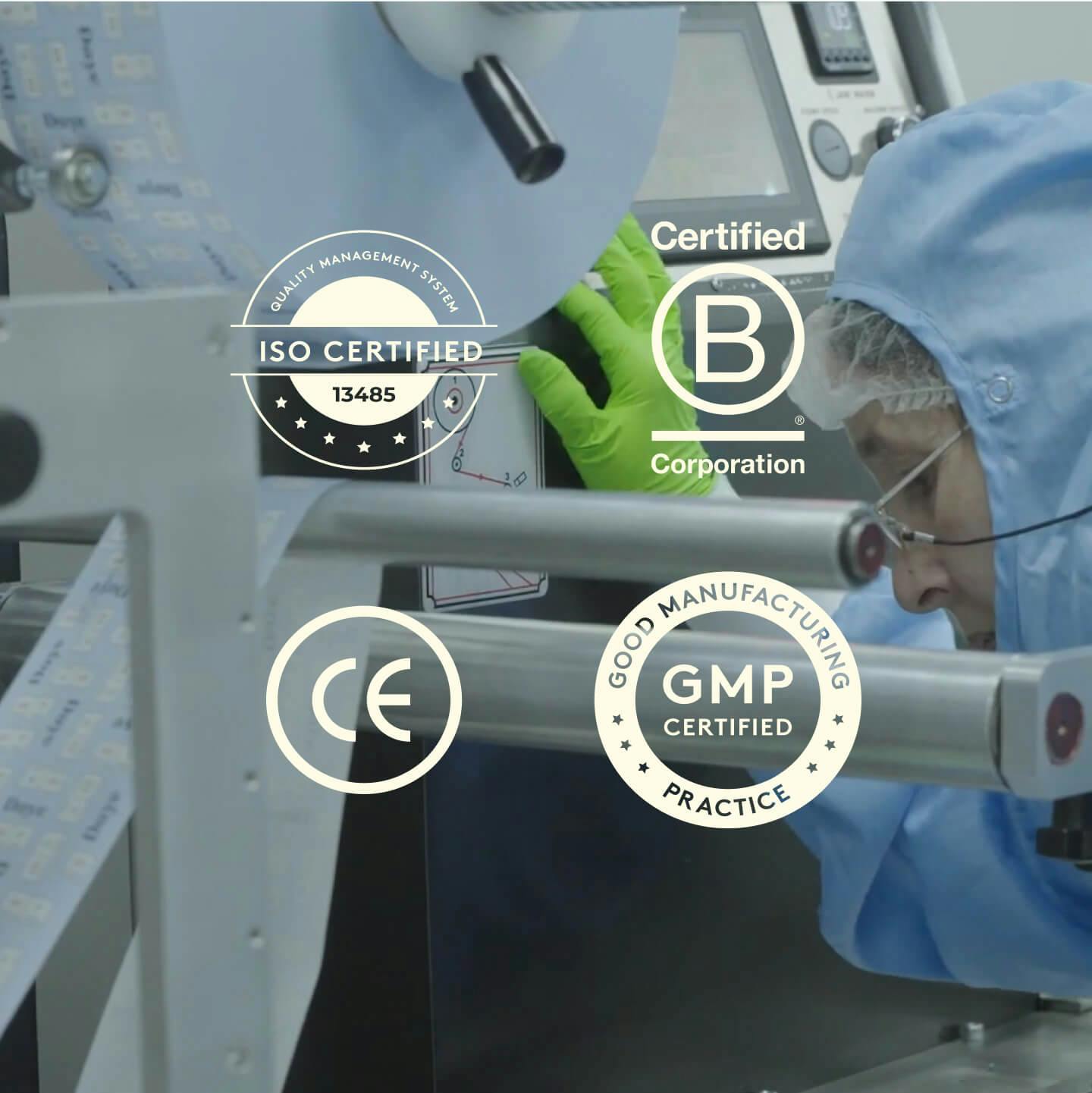
Our tests are validated to the highest industry standards for molecular testing, including UKAS certification. We use an advanced QR code scanning system allows us to track all samples and minimise the risk of manual entry errors. Controls samples are added to each test batch, running alongside patient samples to ensure accuracy of results.
Developed by experts
"HPV screening with tampons empowers women...”
"HPV screening with tampons empowers women to take charge of their health proactively. By seamlessly integrating screening into their routine, we can detect and prevent cervical cancer early.
Developed by experts
We’re raising the standards of gynae products
To guarantee we deliver the safest, most effective products possible, we have independent groups audit Daye to confirm compliance with top industry quality standards.
Choose a screen that works for you
HPV + Microbiome + STI Screen
£277
What we test for:
HPV16
HPV18
12 other high-risk HPV types
Lactobacilli
Common BV related bacteria
Candida
Mycoplasma hominis
Ureaplasma
Gonorrhea
Chlamydia
Trichomoniasis
Mycoplasma genitalium
HPV + Microbiome Screen
£188
What we test for:
HPV16
HPV18
Other high-risk HPV types
Lactobacilli
Common BV related bacteria
Candida
Mycoplasma hominis
Ureaplasma
HPV Screen
What we test for:
HPV16
HPV18
Other high-risk HPV types
See our Founder’s results!
Wondering what we test for, and what results look like? Enter your email below and Valentina, our Founder, will send you her screening report.
By entering your email above you agree to receive marketing emails and promotions from Daye.

Frequently asked questions
Still have questions? Read all of our FAQs.
HPV, or human papillomavirus, is a very common virus that can spread through sexual contact. There are over 100 different strains of HPV, with some considered "high-risk" for causing cancers like cervical cancer if left undetected and untreated.
Testing for high-risk HPV strains is important because the virus often doesn't cause symptoms initially. However, persistent HPV infection can lead to precancerous cervical lesions that may develop into cervical cancer over time if not monitored and treated appropriately.
Regular HPV screening allows for early detection of high-risk strains before pre-cancerous changes occur. This allows for proactive treatment and close monitoring to prevent cervical cancer from developing. HPV testing helps catch potential issues early when they are most treatable and manageable.
The tampon offers a non-invasive, familiar method of obtaining a testing sample that makes the screening experience at home more comfortable and accessible. Further, tampons collect a comprehensive sample from the entirety of the vaginal canal, allowing for greater accuracy compared to traditional methods like swabs. The applicator enables smooth insertion in the vagina, and it also prevents the tampon from being contaminated during insertion.
Using the kit at home is super easy — you just need to insert the provided tampon, wear it for at least 20 minutes (and up to 8 hours), and then put it in the pouches you have received in the kit, and ship it back to us in the same box you received initially. We’ve prepared detailed video instructions which you’ll see after completing the test activation questionnaire in your account.
After your sample reaches our lab, it will be analysed within 5–10 days. You will receive an email notification when your results are ready for review, or if there was an issue with your sample.
We know receiving abnormal results can be worrying, and we want to support you in getting the right care for your situation. While our HPV screening is great for early detection, it's not the best tool for monitoring cervical cell changes. Here's why:
When cell changes are present in your cervix, your healthcare team needs to keep a close eye on these cells specifically - something a HPV test can't do.Even if HPV is no longer detectable in your vaginal canal, the virus may have already become part of your cervical cells, which requires different types of monitoring, including:
- Regular colposcopies (detailed examinations of your cervix)
- Possible biopsies (small tissue samples)
- Close monitoring by your healthcare team
The best thing to do is to continue attending your scheduled cervical screening appointments and follow your healthcare provider's recommended screening schedule.
Our screening works best for people who want to be proactive about their cervical health before any cell changes occur. This includes:
- People between their regular NHS cervical screenings
- Those who find traditional screenings anxiety-inducing and want an at-home option
- People who want to track if their body has cleared a previous HPV infection
If your HPV screening results are negative for high-risk HPV strains, no further action is typically needed beyond continued routine screening and maintaining a healthy vaginal microbiome as a first line of defence against future infections. Through your Daye account, you will be able to connect with vetted expert nurses to discuss your results and next steps.
If your results show the presence of high-risk HPV, don't worry - this does not mean you have cancer. However, it does indicate a need for further evaluation and monitoring. You will find information about the recommended further testing and monitoring in your report.
Daye's HPV screen uses gold-standard PCR tests that detect high-risk HPV strains with 92.8% sensitivity and 100% specificity. We partners with UKAS- accredited labs that test samples to the highest industry standards. The Daye diagnostic tampon allows for convenient collection of a comprehensive sample from the entirety of the vaginal canal, with a significantly lower chance of user error compared to traditional methods.
Right now, we're offering our at-home STI & HPV screening services exclusively in the UK. We know reproductive health doesn't stop at borders though, so we're working hard to bring our tampon-based screening to more countries in the future! If you're outside the UK and interested in our screening service, sign up for our newsletter to be the first to know when we launch in your area.
If you experience any discomfort, unusual symptoms, or adverse reactions while using the tampon for vaginal diagnostics, we recommend removing it immediately and consulting with your healthcare provider. Your health and safety are our utmost priority, and it is essential to address any concerns promptly.
We recommend avoiding the use of the tampon for vaginal diagnostics for a period of six weeks following vaginal birth. This allows ample time for the body to heal and reduces the risk of potential complications. Please consult with your healthcare provider for guidance specific to your individual situation.
In order to take a sample at least 5 days must have passed since your last period ended. There are a few other requirements: no antibiotics or antifungals in the last 30 days, no douching, penetrative vaginal sex, and vaginally applied meds or creams in the 24 hours before. That will ensure your microbiome is in a stable state and that your results are not inconclusive.
In addition to the postpartum period, we advise against using the tampon for vaginal diagnostics if you have any open wounds, sores, or other vaginal irritations. It is essential to prioritize your comfort and well-being, and if you have any concerns, please consult your healthcare provider.
Your kit stays valid for 6 months from purchase date, giving you plenty of time to test when it feels right for you. You can keep the kit at room temperature until then - no special storage needed.
When you are ready to test, just remember these two simple steps:
- Activate your kit in your account on the day you're taking your sample
- Follow the packing instructions and send your sample back to us in the original box - we've already put the prepaid return label on it
Have any questions? Our team at hello@yourdaye.com is always here to help!
Yes, we take your privacy seriously. Your personal information and test results are kept confidential. We follow strict privacy and data protection protocols to ensure your information remains secure.
No, it's best to avoid using the HPV screening tampon during your menstrual period. You can use the tampon at other times during your cycle when you are not menstruating.
While our nurses and vetted specialists can provide valuable insights and guidance, they can't formally diagnose conditions through Daye's platform. Our service is designed to help detect potential issues, but it's not a substitute for an in-person medical diagnosis.
However, we do offer a convenient pharmacy service. We partner with licensed prescribing pharmacists who can review your medical history and screening results. If appropriate, they can provide prescriptions for certain conditions like bacterial vaginosis or thrush, which can then be delivered discreetly to your door. This service offers a convenient way to access treatment for common gynaecological issues. For more complex concerns or if you're unsure, we still recommend following up with a healthcare provider for a comprehensive evaluation. Remember, your health and wellbeing are our top priority, and we're here to empower you with knowledge, resources, and convenient care options when possible.
Our diagnostic tampon screening service is only available to people aged 18 and over. This age requirement is in place due to regulatory reasons and because our service is designed for adults who can legally consent to medical screening and handling of sensitive health data without parental permission. The 18+ requirement ensures we're operating within legal and ethical guidelines for healthcare services.
If you're under 18, we recommend visiting your GP or a local sexual health clinic where appropriate services for young people are available. Many sexual health clinics offer free and confidential services specifically designed for teenagers.
Relevant Research
The vaginal microbiota associates with the regression of untreated cervical intraepithelial neoplasia 2 lesions
2020 • Anita Mitra, David A MacIntyre, George Ntritsos, Ann Smith, Konstantinos K Tsilidis, Julian R Marchesi, Phillip R Bennett, Anna-Barbara Moscicki, Maria Kyrgiou
Emerging evidence suggests associations between the vaginal microbiota (VMB) composition, human papillomavirus (HPV) infection, and cervical intraepithelial neoplasia (CIN); however, causal inference remains uncertain. Here, we use bacterial DNA sequencing from serially collected vaginal samples from a cohort of 87 adolescent and young women aged 16-26 years with histologically confirmed, untreated CIN2 lesions to determine whether VMB composition affects rates of regression over 24 months. We show that women with a Lactobacillus-dominant microbiome at baseline are more likely to have regressive disease at 12 months. Lactobacillus spp. depletion and presence of specific anaerobic taxa including Megasphaera, Prevotella timonensis and Gardnerella vaginalis are associated with CIN2 persistence and slower regression. These findings suggest that VMB composition may be a future useful biomarker in predicting disease outcome and tailoring surveillance, whilst it may offer rational targets for the development of new prevention and treatment strategies.
The vaginal microbiome: A complex milieu affecting risk of human papillomavirus persistence and cervical cancer
2022 • Stephanie Alimena, Joshua Davis, Raina N. Fichorova, Sarah Feldman
The purpose of this review is to describe the existing literature regarding the relationship between the vaginal microbiome, human papillomavirus persistence, and cervical cancer risk, as well as to discuss factors that mediate these relationships. Data suggest that alterations in the vaginal microbiome affect the risk of human papillomavirus infection and persistence, which has downstream effects on cervical dysplasia and cancer risk.
Role of Immunity and Vaginal Microbiome in Clearance and Persistence of Human Papillomavirus Infection
2022 • Lungelo Ntuli, Andile Mtshali, Gugulethu Mzobe, Lenine JP Liebenberg, Sinaye Ngcapu
Recently, diverse vaginal microbiome (associated with bacterial vaginosis) and genital inflammation have emerged as potential drivers of high-risk HPV positivity and disease severity in women. The potential role of these risk factors on HPV recurrence and persistence remains unclear. This article reviews the role of cellular or cytokine response and vaginal microbiome dysbiosis in the clearance, persistence, and recurrence of HPV infection.
Long-term Absolute Risk of Cervical Intraepithelial Neoplasia Grade 3 or Worse Following Human Papillomavirus Infection: Role of Persistence
2010 • Susanne K. Kjær, Kirsten Frederiksen, Christian Munk, Thomas Iftner
HPV16, HPV18, HPV31, and HPV33 infection and especially HPV16 persistence were associated with high absolute risks for progression to high-grade cervical lesions. The results indicate the potential value of genotyping in cervical cancer screening. Given that HPV DNA–negative women retained their low risk of CIN3 or worse for many years, frequent screening of these women may be unnecessary.
Oral cancer risk in relation to sexual history and evidence of human papillomavirus infection
1998 • S M Schwartz, J R Daling, D R Doody, G C Wipf, J J Carter, M M Madeleine, E J Mao, E D Fitzgibbons, S Huang, A M Beckmann, J K McDougall, D A Galloway
Experimental models and analyses of human tumors suggest that oncogenic, sexually transmittable human papillomaviruses (HPVs) are etiologic factors in the development of oral squamous cell carcinoma (SCC). We conducted a population-based, case-control study to determine whether the risk of this cancer is related to HPV infection and sexual history factors. HPV type 16 infection may contribute to the development of a small proportion of oral SCCs in this population, most likely in combination with cigarette smoking.
Efficacy and Acceptability of Self-Collected Medical Grade Tampon as a Novel Vaginal Sample Collection Tool for the Detection of HPV and STIs
2024 • Valentina Milanova, Iva Lazarova, Kalina Mihaylova, Michelle Gomes, Teodora Georgieva, Jan Multmeier
Self-collected tampons demonstrated promising diagnostic accuracy to HCW-collected swabs for HPV and STI detection. The tampon self-collection method was well-accepted and preferred by participants, suggesting its potential as an alternative screening tool, particularly in low-resource settings. Further research with larger and more diverse populations is recommended to validate these findings and inform tampon-based self-collection programs for cervical cancer screening. Randomised controlled trials and comparisons with gold standard methods would enhance validation.
Tampons: A Novel Patient-Administered Method for the Assessment of Genital Human Papillomavirus Infection
1992 • Christopher K. Fairley, Shujun Chen, Sepehr N. Tabrizi, Michael A. Quinn, John J. McNeil, Suzanne M. Garland
Assessment of human papillomavirus (HPV) infection usually requires a speculum examination to collect genital specimens. A technique using tampons as.a patient-administered method for the collection of specimens was studied by dot blot hybridization (HPV types 6, 11, 16, 18, 31, and 33) and polymerase chain reaction (PCR). Tampons and cervical scrapes were collected from 48 consecutivewomen attending a dysplasia clinic. Tampons provided a significantlylarger pellet volume (P < .002) and more DNA (P < .01) than scrapes. There was a close correlation when samples were analyzed for the presence of HPV DNA. Using dot blot hybridization, 8 cervical scrapes (17%) and 9 tampons (19%) were positive for HPV DNA (90% correlation). By PCR, 35 cervical scrapes (73%) and 33 tampons (69%) were positive for HPV DNA (88% correlation). Thus, tampon specimens are an easy method for assessment of genital HPV infection.












